Bioquell SeQure
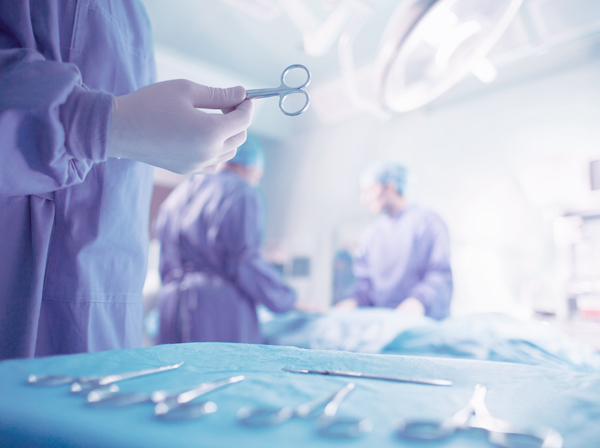
The Bioquell SeQure provides a fixed solution with advanced controls to ensure critical areas can be quickly and easily decontaminated to the fullest extent, emphasising patient and staff safety.
Ideal For:
- Operating theatres
- Reducing Surgical Site Infections (SSIs)
- Hospital pharmacies
- Biocontainment units
- Bioquell Pods
- Patient rooms
Watch Video 
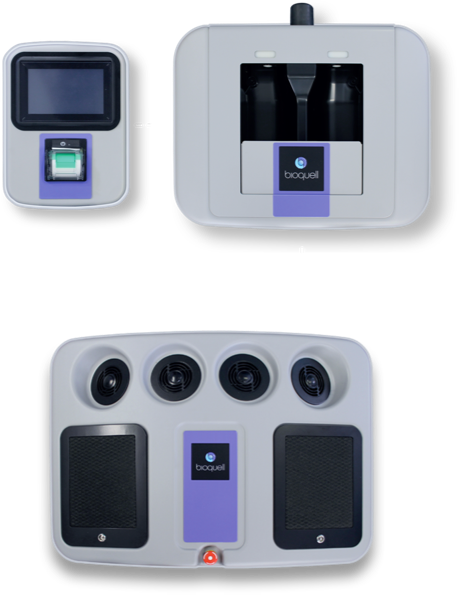
WHY CHOOSE THE Bioquell SeQure
Productive
Fast, effective and automated decontamination cycles
Easy
Simple setup and touchscreen activation to start a cycle

Assured
Eliminate pathogens with validated cycles for consistent and repeatable results

Impactful
More effective than other technologies and manual cleaning; reduces the risk of SSIs from the environment

Integrated
Wall-mounted solution removes setup requirements; saves space

Complete
Every exposed surface disinfected; eliminates pathogens, does not simply reduce them

Watch Bioquell SeQure Video
Preview Key Benefits and Features
APPLICABLE SOLUTIONS
COMPONENTS
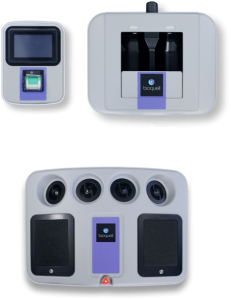
SEQURE COMPONENTS

- 4 integrated fans distribute vapor dynamically
- Automated cycle prediction technology
- Operates at room temperature conditions, safe for heat-sensitive materials

- Easy-to-use, colour touchscreen
- Password Protected programmed decontamination cycles

- Minimizes peroxide wastage with no decanting
- Houses 2 H2O2 bottles (950ml each)
CONSUMABLES & ACCESSORIES

Bioquell Hydrogen Peroxide
Bioquell’s proprietary high-purity aqueous 35% solution for use with the Bioquell SeQure system achieves 99.9999% deactivation of pathogens and a 6-log kill with full traceability and auditing through RFID label technology

Bioquell’s proprietary high-purity aqueous 35% solution for use with the Bioquell SeQure system achieves 99.9999% deactivation of pathogens and a 6-log kill with full traceability and auditing through RFID label technology
Our individually crafted biological indicators made from Geobacillus stearothermophilus endospores ensure reliable results when seeking to validate a 6-log reduction of bioburden from the decontamination process
Installation & Use
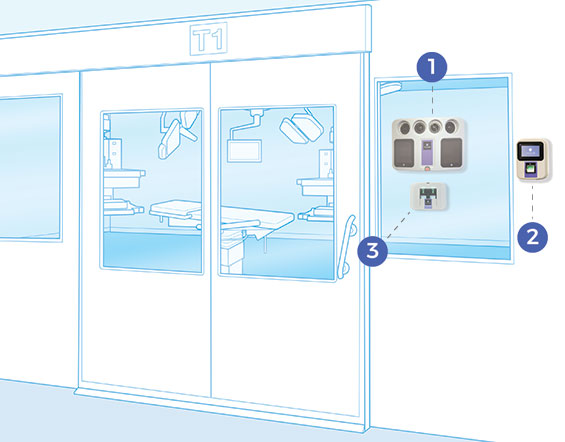
Bioquell SeQure system components are wall-mounted inside or outside of your desired target area for decontamination. It offers a minimal footprint while offering the efficacy and complete room disinfection benefits of Hydrogen Peroxide Vapour.
Whether installed in a new facility or retrofitted into an existing room, each component integrates quickly to minimise downtime while you upgrade to the latest decontamination available to protect your patients and staff.
After installation, a cycle can be run at a moment’s notice. With antibiotic resistance on the rise and dangerous pathogens crossing borders easily, the Bioquell SeQure offers a crucial and immediate defence against these problematic viruses, bacteria, spores and more to keep your patients and staff protected.
FAQs
Will this offer 21-CFR Part 11 compliance?
Yes, we are able to offer an optional data logger for this purpose.
Will installation and validation of the Bioquell SeQure disrupt my workflow?
No. Depending on the volume of the area and the level of equipment contained therein, installation and retrofitting of the Bioquell SeQure can be completed and validated within a week. Our technicians can work during non-operating hours if possible and permitted by the client. Specific timeframes will be given for each project.
Can the Bioquell SeQure connect to my Building Management System?
Yes. Our expert technicians will perform a walkthrough of your cleanroom to ensure your systems are compatible with the Bioquell SeQure for integrated HVAC and automated operation. What’s more, Bioquell experts provide continued support for systems that are already in place, ensuring full BMS connectivity and solutions for the lifetime of your system.
How compact is the Bioquell SeQure?
Vaporiser: 800 x 580 x 160 mm (31.5 x 22.8 x 6.3 in)
Bottle Module: 520 x 550 x 140 mm (20.5 x 21.7 x 5.5 in)
Wired Control Unit: 260 x 350 x 95 mm (10.2 x 13.8 x 3.7 in)
Contact Us
To learn more about how Bioquell can fit your solution, please contact us.
UK Contact
Bioquell UK Ltd
52 Royce Cl,
Andover SP10 3TS, UK
+44 (0)1264 835 835
enquiries@bioquell.com